Thermodynamical Equilibrium and Gasification
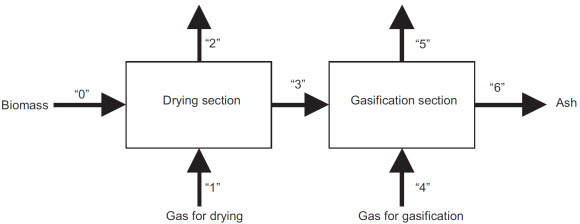
This activity took place in parallel with the thesis of Alexandre Deydier (T7 Modélisation d’un réacteur de gazéification en lit fixe) in partnership with Europlasma company. en collaboration avec la société Europlasma. The goal of this equilibrium model was to predict the composition of a gaz produced by the gasification of an organic load assuming these compostion obeyed the equilibrium assumption. The gasification reactor was composed of two stages: the first one devoted to the drying of the load, and the second one to its gasification.
Sketch of the considered reactor
The completion of the computation requires that the lower heating value, the ultimate and proximate analysis of the biomass be provided to the software. It is also required that characteristices of the gases used for drying and gasification (composition, flowrate and temperature) be provided to the software.
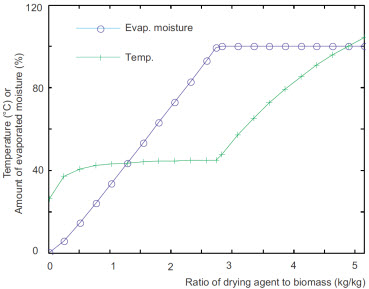
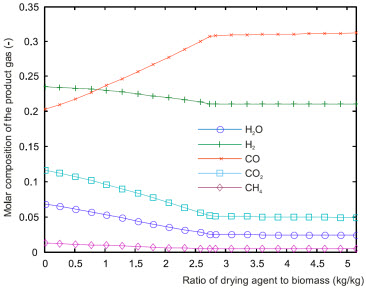
The influence of the mass flow rate of air used for drying is presented in a first set of results.
Influence of the mass flow rate of air used for drying
(referred to the mass flow rate of incoming biomass)
Left: Temperature and moisture content of the biomass leaving
the drying stage
Right: Molar composition of the gas leaving the gasification
stage.
The influence of of the air used for gasification is now reported in a second set of results
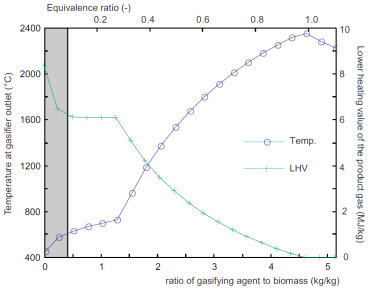
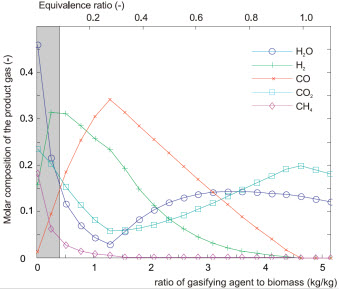
Influence of the mass flow rate of air used for gasification
(Referred to the mass flow rate of incoming biomass)
Left: Temperature and lower hheating value of the product gas.
Right: Molar composition of the product gas.
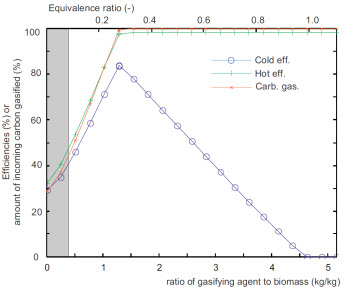
Influence of the mass flow rate of air used for gasification
(Referred to the mass flow rate of incoming biomass)
Evolution of the hot and cold efficiency and of the ratio of
gasified carbon.
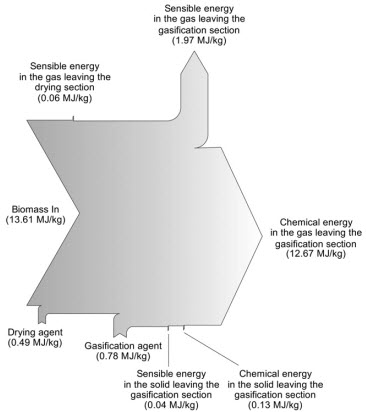
The last presented results show the becoming of the energy held within the biomass as it is processed in the reactor (Sankey) in the nominal case.