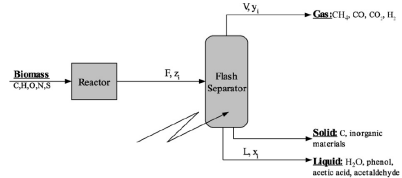
Gasification in Supercritical Water
This activity took place in the frame of the program suported by the National Research Agency (ANR Superbio) for which the goal was to study the opportunity to gasify vinasse (residue from the alcohol industry) in order to feed the boiler producing steam for distillation. The considered process was composed of a gasification reactor and of two phases separator where the expension from the gasification pressure (250 bars) to the atmospheric one was performed.
The first work was devoted to the prediction of the composition of the gas that would leave the reactor if chemcical equilibrium was reached. Associated to a PEng Robinso Equation of State, this assumption allowed estimating the maximal recovery potential and the influence of the operating conditions of the process.
Evolution of the molar composition of the product gas as a function of
the amount of air supplied to the reactor.

Evolution of the Lower Heating value of the gas as well as of the
amount of energy
implied at the reaction and separation stages a a function of the amount of air
supplied to the reactor.

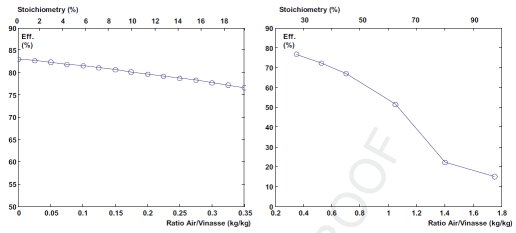
Evolution of the gasification efficiency
as a function of the amount of air suplied to the reactor.
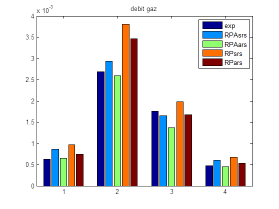
Models involving kinetic information were also derived during this work. They include CSTR and plug flow assumption, with and without secondary reactions (Water Gas shift reaction). This theoretical results were compared to the experimental ones obtained by ICMCB.
Evolution of the gas flow rate as a function of the reaction temperature
Comparaison of numerical and experimental results in the case of
the CSTR (RPA) and Plug Flow reactor (RP)
with (ars) and without (srs) secondary reaction.
Evolution of the molar fraction of CH4 in the product gas as a function
of the reaction temperature
Comparaison of numerical and experimental results in the case of
the CSTR (RPA) and Plug Flow reactor (RP)
with (ars) and without (srs) secondary reaction.